Electroplating is an electrochemical process where a plating layer of metal is deposited on another metal called the substrate. This process can enhance the aesthetic appeal, appearance, conductivity, and corrosion resistance of the substrate. Metals that can be used for electroplating include nickel, gold, silver, copper, chromium, and zinc.
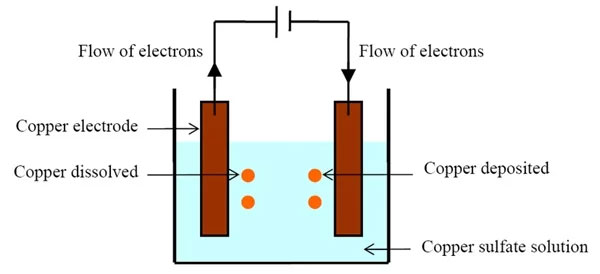
Besides, electroplating metals also help to improve the functionality of the substrate and commonly used in the aerospace, automotive, and jewelry industries. It can be used to improve the acceptance rate of a metal for other protective coatings to prevent rust and wear.